Poster Verena Ohse - GBM Düsseldorf 2023
Grafik: Verena OhseMeeting on compartmentalized redox biology der study group redox biology der Gesellschaft für Biochemie und Molekularbiologie
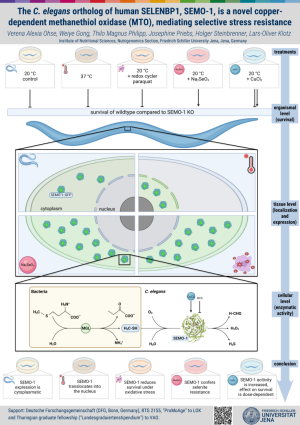
The C. elegans ortholog of human SELENBP1, SEMO-1, is a novel copper-dependent methanethiol oxidase (MTO), mediating selective stress resistance
VA Ohse, W Gong, J Priebs, H Steinbrenner, LO Klotz
Friedrich Schiller University Jena, Institute of Nutritional Sciences, Nutrigenomics Section, Jena, Germany
Abstract
SEMO-1 was previously described as a methanethiol oxidase (MTO) and a pro-aging factor in C. elegans. Here, we demonstrate that Cu is required for SEMO-1 MTO activity: Loss of MTO activity following chelator treatment of recombinant SEMO-1 can be rescued by Cu(II). Growing worms in the presence of Cu or of Cu chelator enhances and lowers MTO activity, respectively. Survival of a semo-1 knockout strain (in relation to wildtype) was assessed following exposure of worms to various stressful stimuli. SEMO-1 confers selective stress resistance in that it impairs survival in the presence of the redox-cycler paraquat, or during heat shock, and renders worms susceptible to acutely toxic Cu concentrations, whereas it confers resistance to selenite and under conditions of chronic Cu exposure. Stress causes changes in SEMO-1 localization and expression: while predominantly cytoplasmic and in hypodermal cells, it is found nuclear and expressed also in intestinal cells upon heat stress.